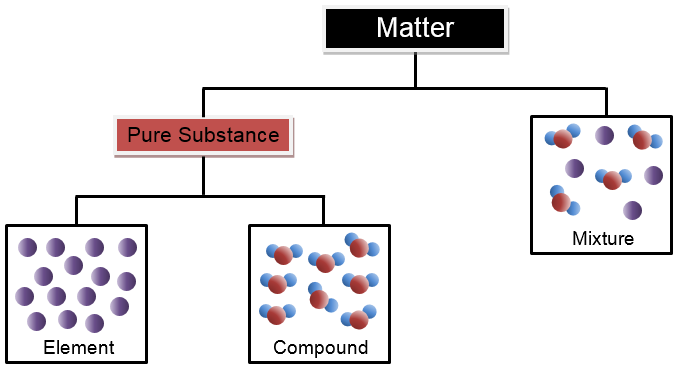
Matter
is anything that has mass and takes up space. All matter exists in one of four states or phases: solid, liquid, gas or plasma.
A
pure substance
cannot be separated into 2 or more substances by physical means. Pure substances have one set of physical and chemical properties. The particles of a pure substance are alike no matter where they are found.
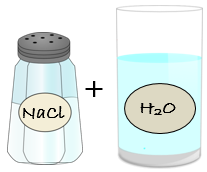
A
mixture
is two or more substances (elements or compounds) that are mixed, but are not chemically combined. Each substance in the mixture keeps their own properties. Saltwater would be an example of a mixture. Saltwater is made up of two compounds: salt and water. Salt is made up of one atom of sodium and one atom of chlorine. The chemical formula for salt is NaCl. Water is made up of two atoms of hydrogen and one atom of oxygen. The chemical formula for water is H2O.
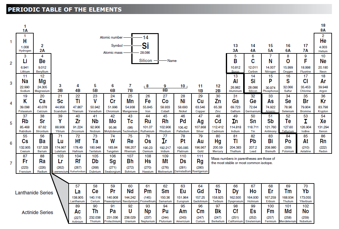
An
element
is matter made up of one type of atom. Elements cannot be broken down into simpler substances. Gold is an example of an element. The simplest form of gold is one atom of gold. A list of the elements can be found on the Periodic Table of Elements. Each element is represented by a unique symbol. Elements are classified as metals, nonmetals, and metalloids.
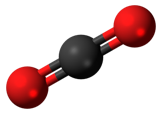
Compounds
are substances that are made up of two or more elements bonded together. Compounds have properties that are different from the elements that make them up. Every compound has a chemical formula. Chemical formulas identify how many atoms of each element are present in a comp
ound. For example, carbon dioxide is a compound. Carbon dioxide is made up of one atom of carbon and two atoms of oxygen. The chemical formula for carbon dioxide is CO2.
script>



