Methane is the main component of natural gas, and its chemical formula is CH4.

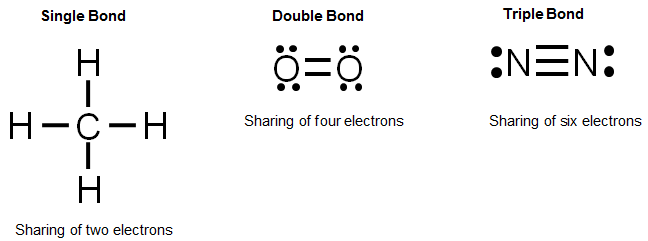
Let's look at the Lewis valence electron dot structures for each of the elements found in methane. Methane contains one carbon atom and four hydrogen atoms.

Remember, carbon needs four electrons to have an octet and complete its valence shell. Hydrogen needs one electron to complete its valence shell.
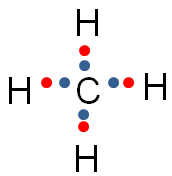
In the image below, the blue circle and red ovals represent the elements sharing electrons in order to complete the valence shells.
Notice that in each of the red ovals around the hydrogen atoms, there are two electrons being shared. (Remember, hydrogen has a completed valence shell with only two electrons.) There are eight electrons in the blue circle around carbon. Eight electrons complete the valence shell of carbon.
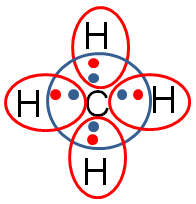
The green lines represent the bond that forms between one of the carbon electrons and the one electron from hydrogen.
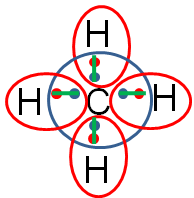
The completed Lewis structure would look like the image below.
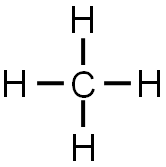
Remember that each line represents two electrons (one electron from the carbon and one from the hydrogen). So, methane is formed by one carbon and four hydrogen creating four single covalent bonds.
The image below is a 3D model of methane. The blue sphere represents the carbon atom and the four red spheres represent the four hydrogen atoms.

Let's look at another example.
The oxygen you breathe is a diatomic molecule that exists as a covalently bonded molecule. Its chemical formula is O2.

Let's look at the Lewis valence electron dot structures for oxygen. Each oxygen atom needs two electrons to make a complete valence shell.

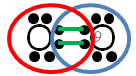
The blue and red ovals represent the elements sharing electrons to complete the valence shell.

Note, inside each oval there are eight electrons, so the valence shell of each oxygen atom is completed by the sharing of these electrons. The green lines represent the bonds between the shared electrons.
The Lewis structure would look like the image below. There are two bonds formed by the sharing of four electrons creating a double covalent bond. The pairs of electrons on each oxygen atom are referred to as lone pairs. Each oxygen atom has two lone pairs of electrons.
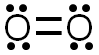
The image below is a 3D model of diatomic oxygen.

Earlier in this lesson, you learned that two, four, or six electrons are shared during covalent bonding. A single covalent bond is the sharing of two electrons, a double covalent bond is the sharing of four electrons, and triple covalent bond is the sharing of six electrons.
Let's look at an example of a triple bond.
Nitrogen gas is also a diatomic molecule. The chemical formula for nitrogen gas is N2. Nitrogen gas makes up about 78 percent of the Earth's atmosphere, but is unusable by organisms.
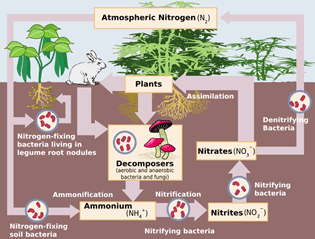
Let's look at the Lewis valence electron dot structures for nitrogen. Each nitrogen atom needs three electrons to complete the valence shell.

Notice that in the Lewis valence electron diagrams, each nitrogen atom has a pair of electrons and three single electrons. The three single electrons will form a triple covalent bond, and a total of six electrons will be shared.
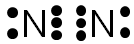
The blue and red ovals represent the elements sharing electrons to complete the valence shell. (Note the single electrons have been moved to the center of the diagram to show the sharing of electrons.)

Note, inside each oval there are eight electrons, so the valence shell of each nitrogen atom is completed by the sharing of these electrons. The green line represents the bond between the shared electrons.

The completed Lewis structure would look like the image below. There are three bonds formed by the sharing of six electrons, so this is a triple bond. Each nitrogen atom has one lone pair of electrons.
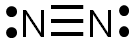
The image below is a 3D model of diatomic nitrogen gas.

Remember there are three types of covalent bonds.