Step 1: Add up the total valence electrons.
Look at all the elements involved in the molecule, and add up the number of valence electrons present. Remember, the valence electrons are the electrons involved in bonding. The group number on the periodic table can determine the number of valence electrons.
CH2O
Element
C:
H: 2(1)=
O:
Total:
Number of Valence Electrons
4
2 (each hydrogen has 1 valence electron, but there are 2 hydrogen atoms, so the total is 2 electrons)
6
12 valence electrons
Step 2: Determine the central atom.
If carbon is in the molecule, carbon is the central atom. If there is no carbon, the least electronegative atom is the central (except hydrogen). Remember that as you go across the periodic table from left to right, electronegativity increases. As you go down the periodic table, electronegativity decreases.
CH2O 12 valence electrons
What would be the central atom?
Step 3: Place non-central atoms around the central atom and connect using lines to represent the bonds.
Each line represents two shared electrons. For each line, subtract two shared electrons from the total number of valence electrons.
CH2O 12 valence electrons
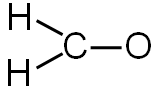
There are three bonds. You will need to subtract six electrons (two for each bond) from the total number of valence electrons.
12 valence electrons − 6 electrons used in bonding = 6 electrons left
Step 4: Distribute the remaining electrons around the non-central atoms for a full valence shell.
If you end up with excess electrons, place them on the central atom.
CAUTION: Hydrogen can only have two electrons. If it is bonded, it cannot hold any more.
12 valence electrons − 6 electrons used in bonding = 6 electrons left
You have six electrons to place on the non-central atoms.
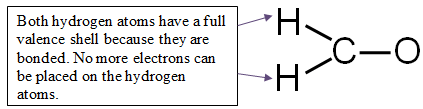
Step 4: Distribute the remaining electrons around the non-central atoms for a full valence shell.
If you end up with excess electrons, place them on the central atom.
CAUTION: Hydrogen can only have two electrons. If it is bonded, it cannot hold any more.
12 valence electrons − 6 electrons used in bonding = 6 electrons left
You have six electrons to place on the non-central atoms.
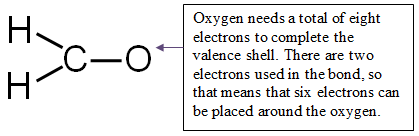
Step 4: Distribute the remaining electrons around the non-central atoms for a full valence shell.
If you end up with excess electrons, place them on the central atom.
CAUTION: Hydrogen can only have two electrons. If it is bonded, it cannot hold any more.
12 valence electrons − 6 electrons used in bonding = 6 electrons left
Place all six electrons around the oxygen.
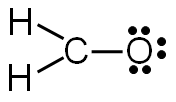
Step 5: Check to make sure all central atoms have a complete valence shell.
If the central atoms do not have a complete valence shell, rearrange some of the lone pairs to make additional bonds.
Does carbon have a complete valence shell?

In this example, carbon does not have a complete valence shell. It only has six electrons, so it needs two more electrons to complete the valence shell.
Step 5: Check to make sure all central atoms have a complete valence shell.
If the central atoms do not have a complete valence shell, rearrange some of the lone pairs to make additional bonds.
If you take away one of the lone pairs of electrons from the oxygen atom and replace it with another bond between the carbon and the oxygen, both carbon and oxygen will have a complete valence shell.
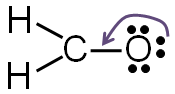
Step 5: Check to make sure all central atoms have a complete valence shell.
If the central atoms do not have a complete valence shell, rearrange some of the lone pairs to make additional bonds.
Now each element in the structure has a complete valence shell.
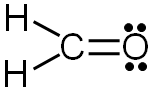
Step 6: Calculate the formal charge of each atom.
Use the following formula to calculate the formal charges:
Formal = # of valence electrons − # of bonds − lone electrons
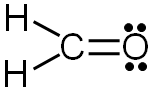
Hydrogen: 1 valence electron − 1 bond − 0 lone electrons = 0 formal charge
Oxygen: 6 valence electrons − 2 bonds − 4 lone electrons = 0 formal charge
Carbon: 4 valence electrons − 4 bonds − 0 lone electrons = 0 formal charge
Since all of the formal charges are zero, our structure is complete.
