Step 1: Add up the total valence electrons.
Look at all the elements involved in the molecule, and add up the number of valence electrons present. Remember, the valence electrons are the electrons involved in bonding. The group number on the periodic table can determine the number of valence electrons. If a molecule is an anion (negatively charged), it has gained electrons. You would then need to add the value of the charge to the number of valence electrons. If the ion is a cation (positively charged), it has lost electrons. You would then need to subtract the value of the charge from the number of valence electrons.
NO3-1
Element
N:
O: 3(6)=
Charge − 1
Total:
Number of Valence Electrons
5
18 (each oxygen has 6 valence electron, but there are 3 oxygen atoms, so the total is 18 electrons)
+1
24 valence electrons
Step 2: Determine the central atom.
If carbon is in the molecule, carbon is the central atom. If there is no carbon, the least electronegative atom is the central (except hydrogen). Remember that as you go across the periodic table from left to right, electronegativity increases. As you go down the periodic table, electronegativity decreases.
NO3-1 24 valence electrons
What would be the central atom?
Step 3: Place non-central atoms around the central atom, and connect the atoms using lines to represent the bonds.
Of the two elements in this molecule, nitrogen is the least electronegative, so it is placed in the center surrounded by the three oxygen atoms.
NO3-1 24 valence electrons
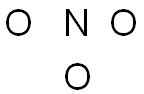
Step 3: Place non-central atoms around the central atom, and connect the atoms using lines to represent the bonds.
Each line represents two shared electrons. For each line, subtract two shared electrons from the total number of valence electrons.
NO3-1 24 valence electrons
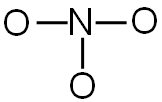
There are three bonds. You will need to subtract six electrons (two for each bond) from the total number of valence electrons.
24 valence electrons − 6 electrons used in bonding = 18 electrons left
Step 4: Distribute the remaining electrons around the non-central atoms for a full valence shell.
If you end up with excess electrons, place them on the central atom.
CAUTION: Hydrogen can only have two electrons. If it is bonded, it cannot hold any more.
24 valence electrons − 6 electrons used in bonding = 18 electrons left
You have 18 electrons to place on the non-central atoms.
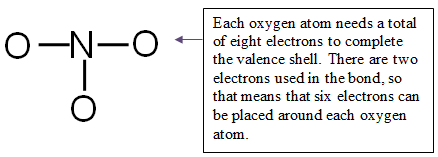
Step 4: Distribute the remaining electrons around the non-central atoms for a full valence shell.
If you end up with excess electrons, place them on the central atom.
CAUTION: Hydrogen can only have two electrons. If it is bonded, it cannot hold any more.
24 valence electrons − 6 electrons used in bonding = 18 electrons left
Place 6 electrons around each oxygen atom for a total of 18 electrons.
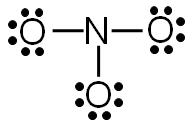
Step 5: Check to make sure all central atoms have a complete valence shell.
If the central atoms do not have a complete valence shell, rearrange some of the lone pairs to make additional bonds.
Does nitrogen have a complete valence shell?

In this example, nitrogen does not have a complete valence shell. It only has six electrons, so it needs two more electrons to complete the valence shell.
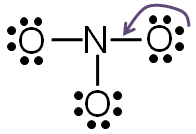
Step 5: Check to make sure all central atoms have a complete valence shell.
If the central atoms do not have a complete valence shell, rearrange some of the lone pairs to make additional bonds.
If you take away one of the lone pairs of electrons from one of the oxygen atom and replace it with another bond between the nitrogen and the oxygen, both nitrogen and oxygen will have a complete valence shell.
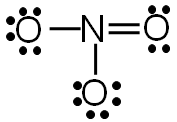
Step 5: Check to make sure all central atoms have a complete valence shell.
If the central atoms do not have a complete valence shell, rearrange some of the lone pairs to make additional bonds.
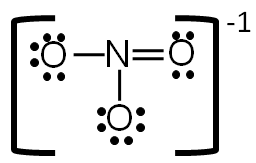
Now each element in the structure has a complete valence shell.
Step 6: Calculate the formal charge of each atom.
Use the following formula to calculate the formal charges:
Formal = # of valence electrons − # of bonds-lone electrons
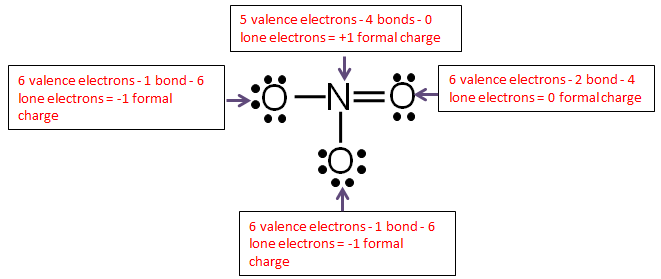
Add up all the formal charges.
| Element | Charge |
| Oxygen | -1 |
| Oxygen | -1 |
| Oxygen | 0 |
| Nitrogen | +1 |
| Total | -1 |
This is as close to formal charges of 0 that we can get. Since the ion has a charge of -1, the sum of formal charges must equal -1. Although creating another double bond seams ideal, as it would make the formal charges for the nitrogen and two oxygen atoms zero, nitrogen cannot have more than eight valence electrons around it. This is because nitrogen is in the second energy level, which only has the "s" and "p" sublevels that can hold a combined total of eight electrons.
In order to show that this is an ion, put brackets around the entire structure and the charge on the outside of the brackets.