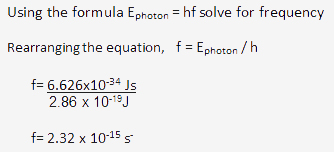We can calculate the energy of electromagnetic radiation two different ways.
We can find out the energy of a photon by using the following equation:
Ephoton = hf
Ephoton = energy of the photon.
h = Planck’s constant which is 6.63 x 10-34 j·s
f = frequency
Here is an example of how to use the equation to find the energy of a photon:
Calculate the energy of a photon of red light of a frequency of 4.6 x 1014 Hz
Calculating the energy of the photon using the equation
Ephoton = hf
Energy E = 6.63 x 10-34 * 4.6 x 1014
=3.0 x 10-19 J
We can find out the energy from a wavelength by using this equation
E = hc/λ
E = Energy
h = Planck’s constant which is 6.63 x 10-34 j·s
c = speed of light which is 3 x 108 m/s
λ = wavelength
![]() Watch this video to see a demonstration of how to use the energy equations using Planck’s constant.
Watch this video to see a demonstration of how to use the energy equations using Planck’s constant.
Source: Energy, Wavelength, Frequency – Part 1, mrscresse, YouTube
Now try out these practice problems. Work the following problems on a scratch sheet of paper. If you get stuck go back and watch the video tutorials again. Once you think you have the correct answer click on the answer box to see the solution.