Source: Texas Education Agency, STAAR Chemistry Reference Materials, Spring 2011
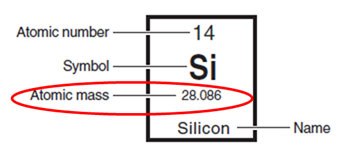
Why isn't the atomic mass in the Periodic Table not a whole number? This is because the Periodic Table shows the average atomic mass for the element. Also, average atomic mass takes into account the very small mass of the electrons of the atoms.

Source: Texas Education Agency, STAAR Chemistry Reference Materials, Spring 2011
Look at the element, silicon, from the periodic table. Notice the atomic mass is 28.086. The atomic mass number of an individual isotope is the number of protons plus the number of neutrons.
P+ + N = Atomic Mass Number (in atomic mass units – amu)
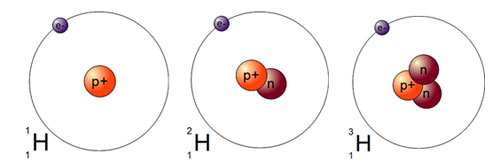
Atoms of the same element will always have the same number of protons (the atomic number) but the number of neutrons may vary. (Remember atoms of the same element with different numbers of neutrons are called isotopes.)
Look at hydrogen, below. Hydrogen has three different isotopes. When we calculate average atomic mass we must consider each isotope and its relative abundance (in percent). Then we calculate the weighted average to find the average atomic mass. The next section will show you how we calculate average atomic mass.