Source: NASA
First let’s look at the difference between radiation and radioactivity.
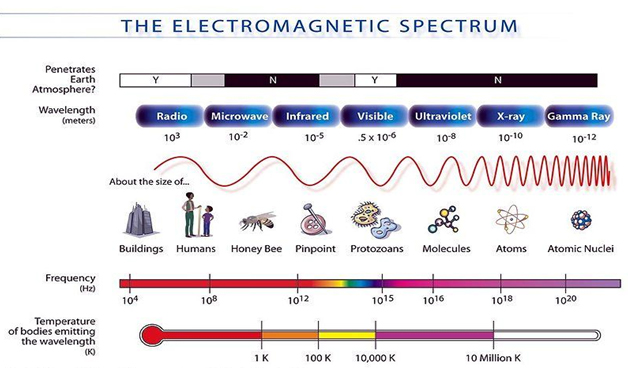
Radiation is a form of energy that moves through space in the form of electromagnetic waves. The microwave used to heat your food and cell phones use radiation. Radio and television waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays are all electromagnetic radiation which makes up the electromagnetic spectrum.
Radioactivity is a spontaneous and random phenomenon that occurs when the nuclei of elements like uranium, give off gamma rays, beta particles (electrons or positrons) and alpha particles (Helium Nuclei). Almost every element has at least one radioactive isotope.
Half-life is the time required for half the nuclei in a sample of a specific isotopic species to undergo radioactive decay.

Source: NASA
Not all types of radiation are harmful or dangerous. Radiation that has sufficient energy to remove electrons from atoms in materials through which the radiation passes is called ionizing radiation. It is only the very short wavelengths, such as x-rays and gamma rays, with the ability to penetrate through clothing, skin and even thin concrete that are dangerous.
Your turn! Refer to the Electromagnetic Spectrum and information above to answer the following questions.