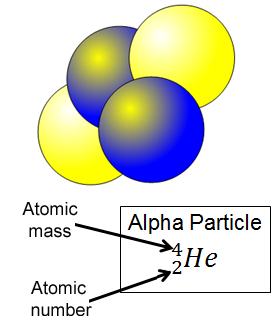
Alpha – a particle (a He nucleus) is emitted causing the atomic mass to reduce by 4 (2 neutrons and 2 protons, and the atomic number to be reduced by 2. View an example of this type of decay.
Symbol : ![]()
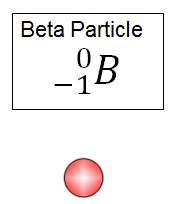
Beta – a particle (either a -b or positron +b) is emitted, which is so small that it causes no change in atomic mass. When negatively charged (-b) it causes a neutron to become a proton, increasing the atomic number by 1. When positively charged (+b) it causes a proton to become a neutron, decreasing the atomic number by 1. View an example of these types of decay.
Symbol: ![]()
Gamma – is an energy packet (photon) which has no mass and no charge. It does not change either the atomic mass or atomic number. View an example of this type of decay.
Symbol: ![]()