When you look at the word “ionic,” what is the first part that you see? ION!
![]()
An ionic bond is formed when the atom of an element releases some of its electrons and reaches a stable electron formation. During the bond, one or more atoms lose electrons. The other atoms gain them in order to produce a noble gas electron configuration. The element that loses the electrons becomes positively charged, and the element that gains the electrons becomes negatively charged.
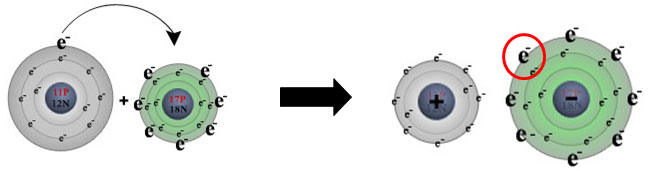
Remember the sodium and chlorine example from section one? Look at the reaction of the movement of these electrons in the diagram below. Sodium (on the left) loses its one valence electron while chlorine (on the right) gains the electron. This movement of electrons results in forming the negatively charged chlorine ion due to the fact that chlorine now has 18 electrons and 17 protons. Likewise, the sodium ion is positively charged due to having 11 protons and only 10 electrons.
![]() Watch the following video to see ionic bonding at the molecular level.
Watch the following video to see ionic bonding at the molecular level.
Source: georectified, ionic bond animation, YouTube
![]() This next video will show you what the reaction looks like in a laboratory setting.
This next video will show you what the reaction looks like in a laboratory setting.
Source: georectified, na and cl go boom, YouTube
Note that one ionic compound is referred to as a formula unit. Ionic compounds are often called salts.
Source: Magnesium, Wikimedia Commons
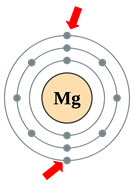
Let’s look at an example. Metals, such as magnesium, usually have one, two, or three electrons in their outermost shell and have lower electronegativities and ionization energies. Therefore, metals are more likely to donate electrons to elements with higher electronegativities and ionization energies. Magnesium has two valence electrons as shown by the red arrows.
Source: Oxygen, Wikimedia Commons
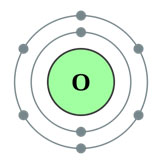
Nonmetals, such as oxygen, have five, six, or seven electrons in their outer shell. Atoms with outer shells that are only partially filled are unstable. More electrons are needed to total eight electrons in the outer shell and complete the octet. Oxygen has six valence electrons, so it needs two more electrons to complete the octet.
Ionic compounds must have an anion and a cation. The positively charged metal cation and negatively charged nonmetallic anion act like the different poles of magnets and are drawn to one another. This difference in charge causes an electrostatic attraction that holds the compound together. In the example below, magnesium gives up its two electrons to oxygen and an ionic bond is formed.
![]() Click on the next button in the interactive below to learn more about ionic bonding.
Click on the next button in the interactive below to learn more about ionic bonding.
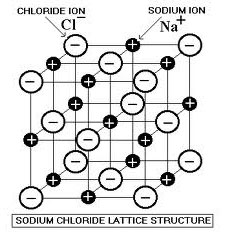
Due to their structure, ionic compounds have particular properties when they are formed. Because of the charged nature of the compound, they create crystal lattice structures, as seen below. In this particular compound, each chloride anion is surrounded by six sodium ions and six chlorides surround each of those six sodium ions. This repeating structure is the reason ionic compounds have many of their properties.
Source: Sodium Chloride Lattice Structure, Doc Browns Chemistry
![]() View the video to see what happens to salt in water on a molecular level.
View the video to see what happens to salt in water on a molecular level.
Source: kosasihiskandarsjah, dissociation of salt, YouTube
![]() Look at the properties of ionic compounds below. Click on each property for more information.
Look at the properties of ionic compounds below. Click on each property for more information.