In order to write the electron dot structures for ionic compounds, let’s first review how to determine the valence electrons using the periodic table.
![]() Look at the Bohr model of the atom below, and then identify the valence electrons by clicking on the correct energy level.
Look at the Bohr model of the atom below, and then identify the valence electrons by clicking on the correct energy level.
Look at the periodic table and compare it to the periodic table from the STAAR Reference Material below.
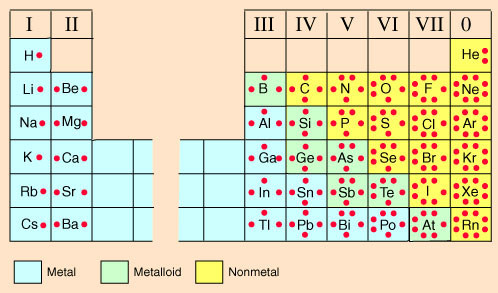
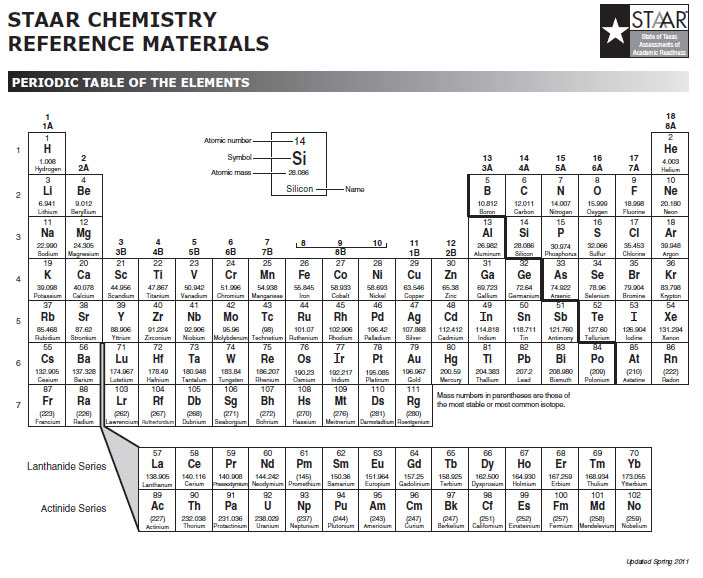
Notice how the group numbers on the STAAR reference chart, 1A, 2A, 3A, etc., match the number of valence electrons as shown on the table with the electron dot diagrams. The exception is helium, HE, which only has one energy level or orbital and contains only two valence electrons, which is a full valence shell. Helium only has two valence electrons. On the table, the element's symbol is in the center of the dot diagram. The dots around the symbol represent the valence electrons. Notice how the dots are not in a circle around the symbol, but form more of a box with up to two electrons on each side and, at the most, eight total valence electrons around the symbol.
It is important to remember that Lewis valence dot diagrams are models that show only the valence electrons. There are often more electrons in the lower energy levels (energy levels that are closer to the nucleus) that are not involved with bonding and, therefore, not shown on an electron dot diagram. These inner electrons can be uncovered if the atom loses all the valence electrons in that valence shell exposing the level below it.
Before you start drawing electron dot formulas of ionic compounds, let’s review how to draw Lewis valence electron dot structures of a single element.
![]()