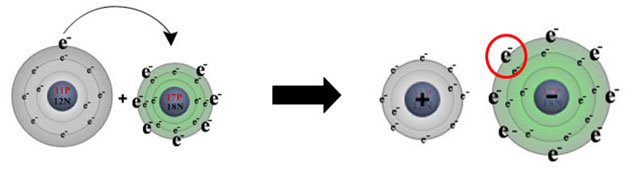
Remember, in ionic bonding one or more atoms lose electrons while other atoms gain electrons in order to produce a noble gas electron configuration or full octet. (Keep in mind that the octet rule is the tendency of atoms in molecules to have eight electrons in their valence shells. It is a general rule but is not followed by all molecules.) The element that loses the electrons becomes a positively charged ion, or cation. The element that gains the electrons becomes negatively charged ion called an anion. The diagram below shows the transfer of one electron from sodium to chlorine.

Ionic bonds occur between metals and nonmetals due to the differences in their electronegativity and ionization energy. Generally, metals have fewer valence electrons and are more likely to lose one, two, or three electrons. Nonmetals have almost full shells and gain three, two, or one electrons. Let’s review what you know about metals and nonmetals.
![]() Directions: Read each statement below and decide if the statement is describing a metal or a nonmetal.
Directions: Read each statement below and decide if the statement is describing a metal or a nonmetal.