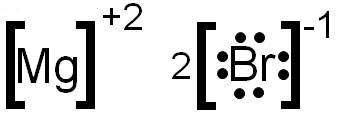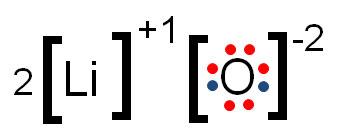
Oxidation numbers are the resulting charge after an atom has given away or taken in electrons in order to have a full valence shell.
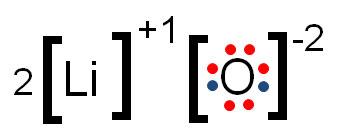
In the example above, oxygen gained two electrons to fill its shell, giving it an oxidation number of -2. Lithium lost one electron and, therefore, its charge was +1, which is also its oxidation number.
Since the elements in a group or family have the same number of valence electrons, they all behave the same way and, in general, have the same oxidation number.
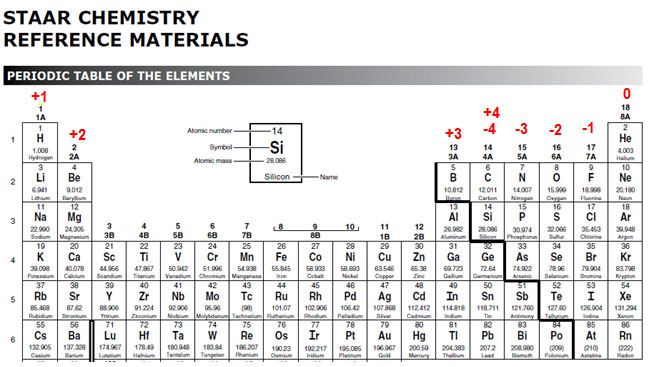
The modified periodic table below shows you the oxidation number of each group. Notice that the transition metals do not have oxidation numbers listed. Also, the noble gases have an oxidation number of zero, and group IV can have an oxidation number of +4 or -4.
![]() Direction: Click on the boxes with the question mark to learn more about these groups.
Direction: Click on the boxes with the question mark to learn more about these groups.
![]() Let’s practice identifying the oxidation numbers. Use the periodic table to complete the chart below. Enter your answers into the boxes. When you have completed all the boxes, click on submit to check your answers.
Let’s practice identifying the oxidation numbers. Use the periodic table to complete the chart below. Enter your answers into the boxes. When you have completed all the boxes, click on submit to check your answers.
Oxidation numbers can be used to help simplify the process of drawing electron dot formulas.
For example, magnesium and bromine form an ionic bond. Magnesium has an oxidation number of +2 and bromine has an oxidation number of -1. The following shows an electron dot formula for the ionic compound that is formed by magnesium and bromine. The oxidation numbers have been added, as superscripts, to each element symbol.
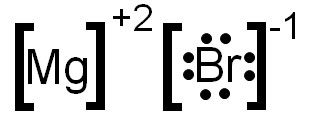
Notice that the overall charge of the compound does not add to zero. Remember, in an ionic compound the oxidation numbers must add to zero. You need to add another bromine to make the oxidation numbers add to zero. The image below shows the correctly written electron dot formula.