
Source: John Dalton, Wikimedia commons, Astrochemist
It was the English scientist John Dalton that studied the properties of gas mixtures as they relate to pressure and developed Dalton's Law. Dalton's Law states: The total pressure of a gas mixture equals the sum of the partial pressures that make up the mixture providing that the gasses do not chemically react with one another. Units may be in torrs, atmospheres, pascals, kilopascals, or other unit of pressure.

Source: John Dalton, Wikimedia commons, Astrochemist
Dalton found that the total pressure of mixed gases is equal to the sum of their individual pressures, provided the gases do not react (notice that the volume is constant).
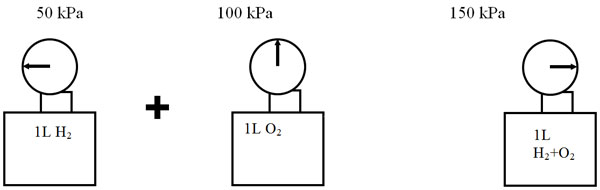
Total pressure for this example = 50 kPa H2 + 100 kPa O2 = 150 kPa H2+O2
Mathematically expressed: Ptotal = P1 + P2 + P3 + ...
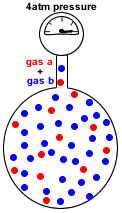
A container of fixed volume at constant temperature holds a mixture of gas a and gas b at a total pressure of 4atm.
The total pressure in the container is proportional to the number of gas particles.
Source: Dalton's Law of partial pressure, Aus-e-tute
More gas particles = greater pressure.
Less gas particles = lower pressure.
If each dot represents 1 mole of gas particles, then there are 48 moles of gas particles in this container exerting a total pressure of 4atm.
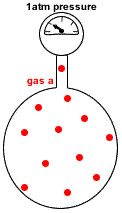
Imagine the container with no particles of gas b.
Only particles of gas a are present in the same container at the same temperature.
Now the container holds only 12 moles of gas particles instead of the 48 moles of gas particles it originally contained.
Since pressure is proportional to the number of gas particles, the pressure exerted by gas a = 12mol ÷ 48mol * 4atm = 1atm
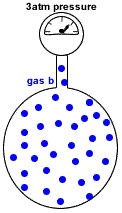
Imagine the container with no particles of gas a.
Only particles of gas b are present in the same container at the same temperature.
Now the container holds only 36 moles of gas particles instead of the 48 moles of gas particles it originally contained.
Since pressure is proportional to the number of gas particles, the pressure exerted by gas b = 36mol ÷ 48mol * 4atm = 3atm
The total pressure in a gas mixture is the sum of the partial pressures of each individual gas.
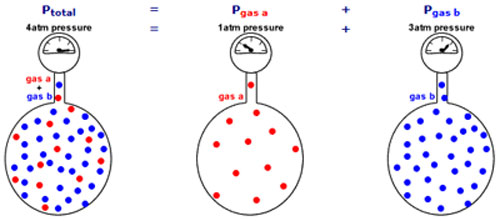
Source: Daltons Law of partial pressure, Aus-e-tute
The first container shows the mixture of gas A and gas B.
Gas A represents 25% of the total pressure (4atm * 25% = 1 atm)
Gas B represents 75% of the total pressure (4atm * 75% = 3 atm)