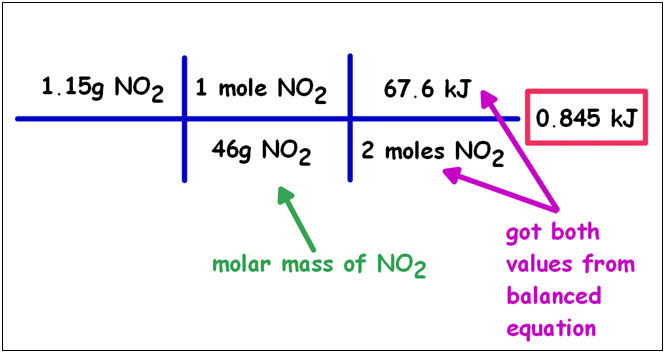
If you have a balanced equation you can convert between mass and energy using dimensional analysis.
Calculate the heat associated with the decomposition of 1.15g of NO2(g) according to the equation:
N2(g) + 2O2(g) → 2NO2(g) ΔH = +67.6 kJ

Try this one on your own:
Calculate the mass of ethane, C2H6, which must be burned to produce 100kJ of heat.
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l) ΔH = -3120kJ
Is this reaction endothermic or exothermic?