
In the last example, solid sugar was dissolved into water. But what is the effect of temperature when you are dissolving a gas into water? Click on the images to check your answer.




Dissolved gases play an important role in nature. Fish use the oxygen dissolved in water for respiration. If oxygen isn’t dissolved, the fish will die. Look at the graph below that shows the relationship between temperature and the amount of gas dissolved in water.
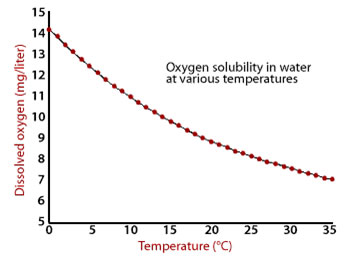
In your notes, answer the following questions about the graph above.
Interactive popup. Assistance may be required.
0°C
Interactive popup. Assistance may be required.
The amount of O2 that is able to dissolve decreases as the temperature increases.
Interactive popup. Assistance may be required.
Temperature has the opposite effect on the solubility of solids and gases. Increasing temperature increases the solubility of solids, but it decreases the solubility of gases. The graph of temperature and solubility for a solid would slope in the opposite direction than the one above.
![]() Practice! Complete the card sort by clicking on the correct column for the factors that affect solubility into the correct place.
Practice! Complete the card sort by clicking on the correct column for the factors that affect solubility into the correct place.
Sources for images used in this section, as they appear, from top to bottom: