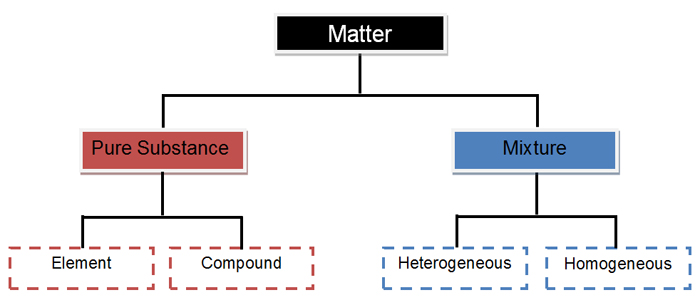
Matter
Matter is anything that has mass and takes up space. All matter exists in one of four states or phases: solid, liquid, gas or plasma. Matter can be classified as an element, compound or mixture according to its composition.
Pure Substance
A pure substance cannot be separated into 2 or more substances by physical means. Pure substances have one set of physical and chemical properties. The particles of a pure substance are alike no matter where they are found. There are two types of pure substances: elements and compounds.
Mixture
A mixture is a combination of two or more pure substances that are not chemically combined. Mixtures are held together by physical forces and therefore can be separated physically.
Element
An element is a pure substance made entirely of one type of atom. Elements cannot be separated into simpler substance by physical or chemical means. Each element has a unique set of properties that can be used to identify that element. Information about the elements can be found on the Periodic Table of Elements. There are currently 118 known elements. Ninety elements are found in nature and the rest are man-made in a laboratory.
Compound
A compound is a pure substance whose smallest unit is made up of atoms of two or more elements joined by chemical bonds. Compounds have different properties than the individual elements that make up the compound. Compounds can only be separated by chemical means.
Heterogeneous
A heterogeneous mixture is a mixture of physically distinct substances with different properties. They do not have the same composition and properties. You can see the different parts of a heterogeneous mixture.
Homogeneous
A homogeneous mixture is a combination of substances that has uniform composition and properties. In a homogeneous mixture the parts are visually indistinguishable from each other meaning you cannot see the different parts.